Unsupervised Pre-Training on Patient Population Graphs for Patient-Level Predictions
基于患者群体图的无监督预训练在患者级别预测中的应用
Abstract. Pre-training has shown success in different areas of machine learning, such as Computer Vision (CV), Natural Language Processing (NLP) and medical imaging. However, it has not been fully explored for clinical data analysis. Even though an immense amount of Electronic Health Record (EHR) data is recorded, data and labels can be scarce if the data is collected in small hospitals or deals with rare diseases. In such scenarios, pre-training on a larger set of EHR data could improve the model performance. In this paper, we apply unsupervised pre-training to heterogeneous, multi-modal EHR data for patient outcome prediction. To model this data, we leverage graph deep learning over population graphs. We first design a network architecture based on graph transformer designed to handle various input feature types occurring in EHR data, like continuous, discrete, and time-series features, allowing better multi-modal data fusion. Further, we design pre-training methods based on masked imputation to pre-train our network before fine-tuning on different end tasks. Pre-training is done in a fully unsupervised fashion, which lays the groundwork for pre-training on large public datasets with different tasks and similar modalities in the future. We test our method on two medical datasets of patient records, TADPOLE and MIMIC-III, including imaging and non-imaging features and different prediction tasks. We find that our proposed graph based pretraining method helps in modeling the data at a population level and further improves performance on the fine tuning tasks in terms of AUC on average by $4.15%$ for MIMIC and $7.64%$ for TADPOLE.
摘要。预训练已在机器学习的不同领域展现出成效,如计算机视觉(CV)、自然语言处理(NLP)和医学影像。然而,其在临床数据分析中的应用尚未得到充分探索。尽管电子健康记录(EHR)数据被大量记录,但如果数据来自小型医院或涉及罕见疾病,数据和标签可能仍然稀缺。在此类场景中,基于更大规模EHR数据的预训练有望提升模型性能。本文针对异质多模态EHR数据,采用无监督预训练方法进行患者预后预测。为建模此类数据,我们利用基于群体图的图深度学习技术。首先设计了一种基于图Transformer的网络架构,可处理EHR数据中各类输入特征类型(如连续型、离散型和时序特征),实现更优的多模态数据融合;其次设计了基于掩码填补的预训练方法,用于在不同下游任务微调前预训练网络。预训练全程采用无监督方式,为未来在大型公共数据集上开展跨任务、同模态预训练奠定基础。我们在TADPOLE和MIMIC-III两个包含影像与非影像特征的患者记录医疗数据集上测试方法,涉及不同预测任务。实验表明,基于图的预训练方法能有效建模群体层面数据,使微调任务平均AUC指标提升:MIMIC提升4.15%,TADPOLE提升7.64%。
1 Introduction
1 引言
Enormous amounts of data are collected on a daily basis in hospitals. Nevertheless, labeled data can be scarce, as labeling can be tedious, time-consuming, and expensive. Further, in small hospitals and for rare diseases, only little data is accumulated [15]. The ability to leverage the large body of unlabeled medical data to improve in prediction tasks over such small labeled datasets could increase the confidence of AI models for clinical outcome prediction. Unsupervised pre-training was shown to be useful to exploit unlabeled data in NLP [20,4],
医院每天都会收集大量数据。然而,标注数据可能十分稀缺,因为标注过程往往繁琐、耗时且成本高昂。此外,小型医院和罕见疾病领域积累的数据量通常有限 [15]。若能利用海量未标注医疗数据来提升小规模标注数据集上的预测任务性能,将显著增强AI模型在临床结果预测中的可信度。无监督预训练已被证明能有效挖掘NLP领域未标注数据的价值 [20,4]。
CV [18,1] and medical imaging [16,2]. However, for more complex clinical data, it is not explored enough. Some works study how to pre-train BERT [4] over EHR data of medical diagnostic codes. They pre-train with modified masked language modeling and, in one case, a supervised prediction task. The targeted downstream tasks lie in disease and medication code prediction [23,11,21]. McDermott et al. [14] propose a pre-training approach over heterogeneous EHR data, including e.g. continuous lab results. They create a benchmark with several downstream tasks over the eICU [19] and MIMIC-III [7] datasets and present two baseline pre-training methods. Pre-training and fine-tuning are performed over single EHRs from ICU stays with a Gated Recurrent Unit.
计算机视觉 [18,1] 和医学影像 [16,2] 领域已有相关研究。然而,针对更复杂的临床数据,探索仍显不足。部分研究探讨了如何在医疗诊断编码的电子健康记录 (EHR) 数据上预训练 BERT [4],采用改进的掩码语言建模方法进行预训练,其中一项研究还结合了监督预测任务。这些研究的下游任务目标集中在疾病和药物编码预测 [23,11,21]。McDermott 等人 [14] 提出了一种针对异构 EHR 数据(包括连续实验室结果等)的预训练方法。他们基于 eICU [19] 和 MIMIC-III [7] 数据集创建了包含多个下游任务的基准测试,并提出了两种基线预训练方法。该方法使用门控循环单元 (Gated Recurrent Unit) 对 ICU 住院期间的单一 EHR 数据进行预训练和微调。
On the other hand, population graphs have been leveraged in the recent literature to help analyze patient data using the relationships among patients, leading to clinically semantic modeling of the data. During unsupervised pre-training, graphs allow learning representations based on feature similarities between the patients, which can then help to improve patient-level predictions. Several works successfully apply pre-training to graph data in different domains like molecular graphs and on common graph benchmarks. Proposed pre-training strategies include node level tasks, like attribute reconstruction, graph level tasks like property prediction, or generative tasks such as edge prediction [5,22,27,6,12]. To the best of our knowledge, no previous work applied pre-training to population graphs.
另一方面,近期研究利用人群图 (population graphs) 通过患者间的关系辅助分析临床数据,实现对数据的临床语义建模。在无监督预训练阶段,图结构能基于患者间的特征相似性学习表征,从而提升患者层面的预测效果。多项研究已成功将预训练应用于分子图等不同领域的图数据及常见图基准测试。提出的预训练策略包括节点级任务(如属性重构)、图级任务(如性质预测)以及生成式任务(如边预测)[5,22,27,6,12]。据我们所知,尚无研究将预训练应用于人群图。
In this paper, we propose a model capable of pre-training for understanding patient population data. We choose two medical applications in brain imaging and EHR data analysis on the public datasets TADPOLE [13] and MIMICIII [7] for Alzheimer’s disease prediction [17,9,3] and Length-of-Stay prediction [26,24,14]. The code is available at https://github.com/ChantalMP/UnsupervisedPre-Training-on-Patient-Population-Graphs-for-Patient-Level-Predictions.
本文提出了一种能够预训练以理解患者群体数据的模型。我们在公共数据集TADPOLE [13]和MIMICIII [7]上选择了脑成像和电子健康记录(EHR)数据分析两个医疗应用,分别用于阿尔茨海默病预测[17,9,3]和住院时长预测[26,24,14]。代码可在https://github.com/ChantalMP/UnsupervisedPre-Training-on-Patient-Population-Graphs-for-Patient-Level-Predictions获取。
Contribution: We develop an unsupervised pre-training method to learn a general understanding of patient population data modeled as graph, providing a solution to limited labeled data. We show significant performance gains through pre-training when fine-tuning with as little as $1%$ and up to $100%$ labels. Further, we propose a (graph) transformer based model suitable for multi-modal data fusion. It is designed to handle various EHR input types, taking static and time-series data and continuous as well as discrete numerical features into account.
贡献:我们开发了一种无监督预训练方法,用于学习以图结构建模的患者群体数据的通用理解,为解决标注数据有限的问题提供了方案。实验表明,在使用少至1%到多达100%的标注数据进行微调时,预训练能带来显著的性能提升。此外,我们提出了一种基于Transformer的模型,适用于多模态数据融合。该模型设计用于处理各类电子健康记录(EHR)输入,同时考虑静态与时序数据、连续及离散数值特征。
2 Method
2 方法
Let $\mathbf{D}$ be a dataset composed of the EHR data of $N$ patients. The $i^{t h}$ record is represented by $\mathbf{r_{i}}\subseteq[\mathbf{d_{i}},\mathbf{c_{i}},\mathbf{t_{i}}]$ with static discrete features $\mathbf{d}{\mathbf{i}}\in\mathbb{N}^{D}$ , continuous features $\mathbf{c_{i}}\in\mathbb{R}^{C}$ , and time-series features $\mathbf{t_{i}}\in\mathbb{R}^{S\times\tau}$ , where $\tau$ denotes the length of the time-series. For every downstream task $T$ , labels $\mathbf{Y}\in\mathbb{N}^{L}$ are given for $\mathrm{L}$ classes. The task is to predict the classes for the test set patients given all features. Towards this task, we propose to use patient population graphs. Unlike in non-graph-based methods, the model can exploit similarities between patients to better understand the EHR data at patient and population level. Further, unlike conventional graph neural networks, graph transformers allow flexible attention to all nodes, learning which patients are relevant for a task. This would be most apt for learning over population EHR data. Our pipeline consists of two steps. 1) Unsupervised pre-training and 2) Fine-tuning. Unsupervised pre-training enables understanding of general EHR data and disorder progression by training the model for masked imputation task. This understanding can help learn downstream tasks better despite limited labeled data.
设 $\mathbf{D}$ 为一个由 $N$ 名患者的电子健康记录(EHR)数据组成的数据集。第 $i^{t h}$ 条记录表示为 $\mathbf{r_{i}}\subseteq[\mathbf{d_{i}},\mathbf{c_{i}},\mathbf{t_{i}}]$,其中包含静态离散特征 $\mathbf{d}{\mathbf{i}}\in\mathbb{N}^{D}$、连续特征 $\mathbf{c_{i}}\in\mathbb{R}^{C}$ 以及时间序列特征 $\mathbf{t_{i}}\in\mathbb{R}^{S\times\tau}$,其中 $\tau$ 表示时间序列的长度。对于每个下游任务 $T$,给定 $\mathrm{L}$ 个类别的标签 $\mathbf{Y}\in\mathbb{N}^{L}$。该任务的目标是根据所有特征预测测试集患者的类别。为此,我们提出使用患者群体图。与非基于图的方法不同,该模型可以利用患者之间的相似性,更好地在患者和群体层面理解EHR数据。此外,与传统图神经网络不同,图Transformer可以灵活关注所有节点,学习哪些患者与任务相关。这最适合于基于群体EHR数据的学习。我们的流程包含两个步骤:1) 无监督预训练;2) 微调。无监督预训练通过训练模型完成掩码填补任务,实现对EHR数据和疾病进展的一般性理解。这种理解有助于在标记数据有限的情况下更好地学习下游任务。
Graph Construction For each node pair with records $r_{i}$ and $r_{j}$ , we calculate a similarity score $S(r_{i},r_{j})$ between the node features. We use L2 distance for continuous and absolute matching for discrete features. As graph construction is not our focus, we choose the most conventional method using k-NN selection rule. We set k=5 to avoid having many disconnected components and very densely connected regions (see supplementary material). A detailed description of the graph construction per dataset follows in the experiment section.
图构建 对于每对包含记录$r_{i}$和$r_{j}$的节点,我们计算节点特征之间的相似度得分$S(r_{i},r_{j})$。连续特征采用L2距离,离散特征采用精确匹配。由于图构建并非本文重点,我们选用最常规的k近邻(k-NN)选择规则,设定k=5以避免产生过多不连通组件和过度稠密连接区域(详见补充材料)。各数据集的具体图构建方法将在实验部分详述。
Model Architecture Our model consists of an encoder and decoder. The encoder comprises a data embedding module and a graph transformer module explained later. We design the encoder to handle various input data types. The decoder is a simple linear layer capable of capturing the essence of features inclined towards a node-level classification task. Figure 1 shows an overview of our model architecture.
模型架构
我们的模型由编码器和解码器组成。编码器包含数据嵌入模块和后续介绍的图Transformer模块。我们设计的编码器能够处理多种输入数据类型。解码器是一个简单的线性层,能够捕捉偏向节点级分类任务的特征本质。图1展示了我们模型架构的概览。
Data embedding module: Following the conventional Graphormer [25], we process discrete input features by an embedding layer, followed by a summation over the feature dimension, resulting in embedded features $\mathbf{d}{\mathbf{i}}^{\prime}\in\mathbb{R}^{D^{\prime}}$ , where $D^{\prime}$ is the output feature dimension. While Graphormer is limited to static, discrete input features only, we improve upon Graphormer to support also static, continuous input features, which are processed by a linear layer resulting in the embedding vector $\mathbf{c_{i}^{\prime}}\in\mathbb{R}^{C^{\prime}}$ . The third branch of our data embedding module handles timeseries input features $\mathbf{t_{i}}\in\mathbb{R}^{S\times\tau}$ with a linear layer, followed by two transformer layers to deal with variable sequence lengths and allow the model to incorporate temporal context. The output is given by $\mathbf{t}{\mathbf{i},\mathbf{h}}^{\prime}\in\mathbb{R}^{E}$ per time-step h. The mean of these embeddings forms the final time-series embeddings $\mathbf{t}{\mathbf{i}}^{\prime}\in\mathbb{R}^{S^{\prime}}$ . The feature vectors $\mathbf{d_{i}^{\prime},c_{i}^{\prime}}$ and $\mathbf{t_{i}^{\prime}}$ are concatenated to form the final node embeddings $n_{i}\in\mathbb{R}^{F}$ , where $\begin{array}{r}{\boldsymbol{F}=\sum_{F_{k}\subset[D^{\prime},C^{\prime},S^{\prime}]}F_{k}}\end{array}$ , for each of the $N$ nodes.
数据嵌入模块:遵循传统Graphormer [25]的做法,我们通过嵌入层处理离散输入特征,随后沿特征维度求和得到嵌入特征$\mathbf{d}{\mathbf{i}}^{\prime}\in\mathbb{R}^{D^{\prime}}$,其中$D^{\prime}$为输出特征维度。Graphormer仅支持静态离散输入特征,我们在此基础上改进以支持静态连续输入特征,这类特征通过线性层处理生成嵌入向量$\mathbf{c_{i}^{\prime}}\in\mathbb{R}^{C^{\prime}}$。该模块的第三分支通过线性层处理时序输入特征$\mathbf{t_{i}}\in\mathbb{R}^{S\times\tau}$,再经两个Transformer层处理可变序列长度并融入时序上下文信息,最终输出每个时间步h的$\mathbf{t}{\mathbf{i},\mathbf{h}}^{\prime}\in\mathbb{R}^{E}$。这些嵌入的均值构成最终时序嵌入$\mathbf{t}{\mathbf{i}}^{\prime}\in\mathbb{R}^{S^{\prime}}$。将特征向量$\mathbf{d_{i}^{\prime},c_{i}^{\prime}}$与$\mathbf{t_{i}^{\prime}}$拼接后形成最终节点嵌入$n_{i}\in\mathbb{R}^{F}}$,其中$\begin{array}{r}{\boldsymbol{F}=\sum_{F_{k}\subset[D^{\prime},C^{\prime},S^{\prime}]}F_{k}}\end{array}$,共生成$N$个节点的嵌入。
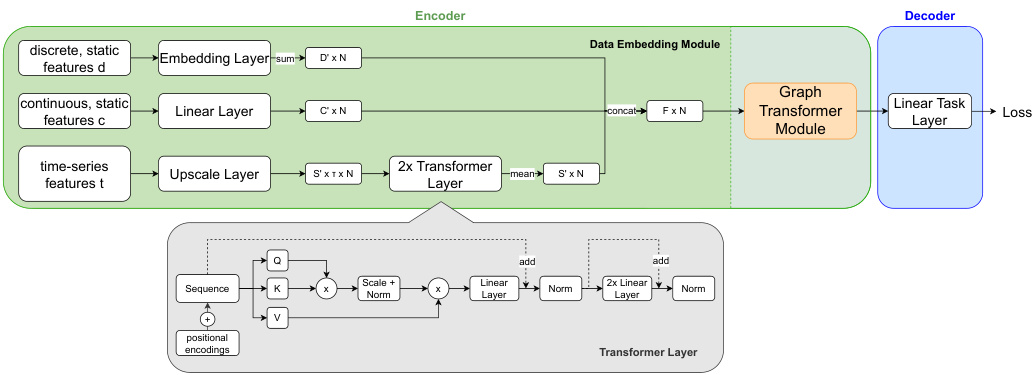
Fig. 1. Overview of the proposed architecture. All input features are combined into one node embedding, applying transformer layers to enhance the time-series features. The upscale layer for time-series features is a linear layer for continuous and an embedding layer followed by a summation over the feature dimension for discrete features. The resulting graph is processed by several Graphormer layers and a linear task layer.
图 1: 提出的架构概览。所有输入特征被合并为一个节点嵌入 (node embedding),应用 Transformer 层来增强时间序列特征。时间序列特征的上采样层对连续特征采用线性层,对离散特征采用嵌入层后沿特征维度求和。生成的图会经过多个 Graphormer 层和一个线性任务层的处理。
Graphormer Module: The backbone of our model comprises multiple graph transformer layers [25]. Graphormer uses attention between all nodes in the graph. To incorporate the graph structure, structural encodings are used, which encode in and out degrees of the nodes, the distance between nodes, and edge features. Pre-training and Fine-Tuning: We propose an unsupervised pre-training technique on the same input features as for downstream tasks, but without using labels $\mathbf{Y}$ . Instead, we randomly mask a fixed percentage of feature values for every record $\mathbf{r_{i}}$ and optimize the model to predict these values. For all methods masking is performed by replacing certain feature values with a fixed value called ’masked token’ for discrete features and with zero for continuous features. For time-series features, we further add a binary column per feature to the input vector, that encodes which hours in the time-series are masked. We optimize the model using (binary) cross entropy loss for discrete and mean squared error loss for continuous features. A model for fine-tuning is initialized using the encoder weights learned during pre-training and random weights for the decoder. Then the model is fine-tuned for the task $T$ .
Graphormer模块:
我们的模型主干由多个图Transformer层[25]构成。Graphormer通过计算图中所有节点间的注意力机制运作。为融入图结构信息,模型采用结构编码技术,对节点的入度/出度、节点间距离及边特征进行编码。
预训练与微调:
我们提出一种无监督预训练技术,其输入特征与下游任务相同,但不使用标签$\mathbf{Y}$。具体实现时,对每条记录$\mathbf{r_{i}}$随机遮蔽固定比例的特征值,并通过模型预测这些值来优化参数。所有方法均采用固定值(离散特征用"遮蔽token",连续特征用零值)替换被遮蔽特征。针对时序特征,我们还在输入向量中为每个特征添加二元列,用于标记被遮蔽的时间点。模型优化时,离散特征采用(二元)交叉熵损失函数,连续特征采用均方误差损失函数。微调模型时,编码器使用预训练获得的权重初始化,解码器则采用随机权重,随后针对任务$T$进行微调。
3 Experiments and Results
3 实验与结果
We use two publicly available medical data sets: TADPOLE [13] and MIMICIII [7]. They differ in size, the targeted prediction task, and the type of input features, allowing comprehensive testing and evaluation of our method.
我们使用了两个公开可用的医学数据集:TADPOLE [13] 和 MIMICIII [7]。它们在数据规模、预测任务目标和输入特征类型上存在差异,能够全面测试和评估我们的方法。
3.1 Datasets description:
3.1 数据集描述:
TADPOLE [13] contains 564 patients from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). We use twelve features, which the TADPOLE challenge claims are informative. They include discrete cognitive test results, demographics, and continuous features extracted from MR and PET imaging, normalized between zero and one. The task is to classify the patients into the groups Cognitive Normal (CN), Mild Cognitive Impairment (MCI), or Alzheimer’s Disease (AD). We only use data from patients’ first visits to avoid leakage of information. Graph Construction: We construct a k-NN graph with k=5, dependent on the mean similarity ( $S$ ) between the features. For the demographics, age, gender and if $f_{i}=f_{j}$ else 0 apoe4, if $|a g e_{i}-a g e_{j}|\leq2$ else 0 $\div3$ where, $f$ =(apoe4, gender). For the cognitive test results $\mathbf{d}{\mathbf{i}}$ (ordinal features), and $\mathbf{c_{i}}$ (continuous imaging features), we calculate the respective normalized L2 distances: $\begin{array}{r}{S_{c o g}(r_{i},r_{j})=\frac{\sum_{f\in\mathbf{d_{i}}}\vert\vert f_{r_{i}}-f_{r_{j}}\vert\vert}{m a x(\mathbf{d_{i}})}}\end{array}$ and $\begin{array}{r}{S_{i m g}(r_{i},r_{j})=s i g(\sum_{f\in\mathbf{c_{i}}}||f_{r_{i}}-f_{r_{j}}||)}\end{array}$ . The overall similarity $S(r_{i},r_{j})$ is then given as mean of $S_{d e m}$ , $S_{c o g}$ and $S_{i m g}$ .
TADPOLE [13] 数据集包含来自阿尔茨海默病神经影像计划 (ADNI) 的564名患者。我们使用了TADPOLE挑战赛声称具有信息量的12个特征,包括离散认知测试结果、人口统计学数据,以及从MR和PET成像中提取并归一化到0到1之间的连续特征。任务是将患者分类为认知正常 (CN)、轻度认知障碍 (MCI) 或阿尔茨海默病 (AD)。为避免信息泄露,我们仅使用患者首次就诊的数据。
图构建:我们基于特征间平均相似度 ( $S$ ) 构建k=5的k-NN图。对于人口统计学数据(年龄、性别和载脂蛋白E4等位基因状态),定义 当 $f_{i}=f_{j}$ 否则为0;若 $|a g e_{i}-a g e_{j}|\leq2$ 则计1否则为0,最终除以3,其中 $f$ =(载脂蛋白E4等位基因状态, 性别)。对于认知测试结果 $\mathbf{d}{\mathbf{i}}$ (序数特征)和 $\mathbf{c_{i}}$ (连续影像特征),我们分别计算归一化L2距离: $\begin{array}{r}{S_{c o g}(r_{i},r_{j})=\frac{\sum_{f\in\mathbf{d_{i}}}\vert\vert f_{r_{i}}-f_{r_{j}}\vert\vert}{m a x(\mathbf{d_{i}})}}\end{array}$ 以及 $\begin{array}{r}{S_{i m g}(r_{i},r_{j})=s i g(\sum_{f\in\mathbf{c_{i}}}||f_{r_{i}}-f_{r_{j}}||)}\end{array}$ 。整体相似度 $S(r_{i},r_{j})$ 取 $S_{d e m}$ 、 $S_{c o g}$ 和 $S_{i m g}$ 的均值。
Pre-Training Configuration: During pre-training on TADPOLE, we randomly mask $30%$ of the medical features (APOE4, cognitive tests, and imaging features) in each sample. The masking ratio of $30%$ was chosen experimentally.
预训练配置:在TADPOLE数据集上进行预训练时,我们对每个样本中30%的医疗特征(APOE4、认知测试和影像特征)进行随机遮蔽。30%的遮蔽比例是通过实验确定的。
MIMIC-III [7] is a large EHR dataset of patient records with various static and time-series data collected over the patient’s stay. We use the pre-processed dataset published by McDermott et al. [14]. It includes 19.7 K patients that are at least 15 years old and stayed 24 hours or more in the ICU. The features include demographics, measurements from bed-side monitoring and lab tests in hourly granularity (continuous), and binary features stating if different treatments were applied in each hour. In total we have 76 features. We use linear interpolation to impute missing measurements. Fine-tuning is evaluated on Length-of-Stay (LOS) prediction as defined in [14]. The input encompasses the first 24 hours of each patient’s stay, and the goal is to predict if a patient will stay longer than three days or not.
MIMIC-III [7] 是一个大型电子健康记录(EHR)数据集,包含患者住院期间收集的各种静态和时序数据。我们使用McDermott等人 [14] 发布的预处理数据集,包含19.7K名年龄≥15岁且在ICU停留≥24小时的患者。特征包括人口统计学指标、床旁监测和实验室检查的小时级测量值(连续型),以及每小时是否实施不同治疗的二元特征,共计76个特征。我们采用线性插值填补缺失测量值。根据[14]的定义,通过在住院时长(LOS)预测任务上进行微调评估:输入为每位患者住院前24小时数据,目标是预测患者是否会住院超过三天。
Graph Construction: It is computationally infeasible to process a graph containing all patients. Thus, we create sub-graphs with 500 patients each, which fit into memory, each containing train, validation and test patients. We split randomly as we do not want to make assumptions on which types of patients the model should see, but learn this via the attention in the graph transformer. Given the time-series of the measurement features $f$ , we form feature descriptors $f_{d}=(m e a n(f),s t d(f),m i n(f),m a x(f))$ per patient and feature, where d equals the 56 measurement features. We then compute the average similarity over all features $f_{d}$ between two patients $r_{i}$ and $r_{j}$ : $\begin{array}{r}{S i m(r_{i},r_{j})=\frac{\sum_{f\in f_{d}}||f_{r_{i}}-f_{r_{j}}||}{|f_{d}|}}\end{array}$ and build a k-NN graph with k=5.
图构建:处理包含所有患者的图在计算上是不可行的。因此,我们创建了每个包含500名患者的子图,这些子图可以放入内存中,每个子图包含训练、验证和测试患者。我们随机拆分,因为我们不想对模型应该看到哪些类型的患者做出假设,而是通过图Transformer中的注意力机制来学习这一点。给定测量特征$f$的时间序列,我们为每名患者和每个特征形成特征描述符$f_{d}=(mean(f),std(f),min(f),max(f))$,其中d等于56个测量特征。然后,我们计算两名患者$r_{i}$和$r_{j}$之间所有特征$f_{d}$的平均相似度:$\begin{array}{r}{Sim(r_{i},r_{j})=\frac{\sum_{f\in f_{d}}||f_{r_{i}}-f_{r_{j}}||}{|f_{d}|}}\end{array}$,并构建一个k=5的k-NN图。
Pre-Training Configuration: On MIMIC-III, we perform masking on the timeseries features from measurement and treatment data. Pre-training is performed over data from the first 24 hours of the patient’s stay. We compute the loss only over measured values, not over interpolated ones. Masking ratios are chosen experimentally. We compare two types of masking:
预训练配置:在MIMIC-III数据集上,我们对测量和治疗数据中的时间序列特征进行掩码处理。预训练仅使用患者住院前24小时的数据。损失函数仅基于实测值计算,不包括插补值。掩码比例通过实验确定。我们比较了两种掩码类型:
Feature Masking (FM): We randomly select $30%$ of the features p